ALCOA Plus Principles are most implemented tools for data integrity in pharmaceutical industries. ‘ALCOA’ is abbreviation of Attributable, Legible, Contemporaneous, Original, and Accurate. In addition,’ ALCOA plus’ principles also recommends that data is also Complete, Consistent, Enduring, and Available.

What is ALCOA Plus?
ALCOA is a set of rules made by the FDA to keep data accurate in manufacturing. It started in 2018 for companies that handle data as regulated by 21 CFR Part 11.
Companies in life sciences use ALCOA plus to show that their products are made safely, follow approved processes and compliance with data Integrity.
ALCOA stands for Attributable, Legible, Contemporaneous, Original, and Accurate. These principles help ensure accurate data and proper processes.
Over time, the ALCOA principles expanded to include Complete, Consistent, Enduring, and Available. These are called ALCOA+ and help with good documentation practices in the pharmaceutical industries.

What are the 9 principles of ALCOA plus?
To grasp the concept more effectively, let’s dive into ALCOA+ and explore each component of this acronym. By doing so, we can gain a clearer understanding of its significance in maintaining data integrity within the manufacturing industry. So, let’s break it down!
Attributable meaning in ALCOA plus
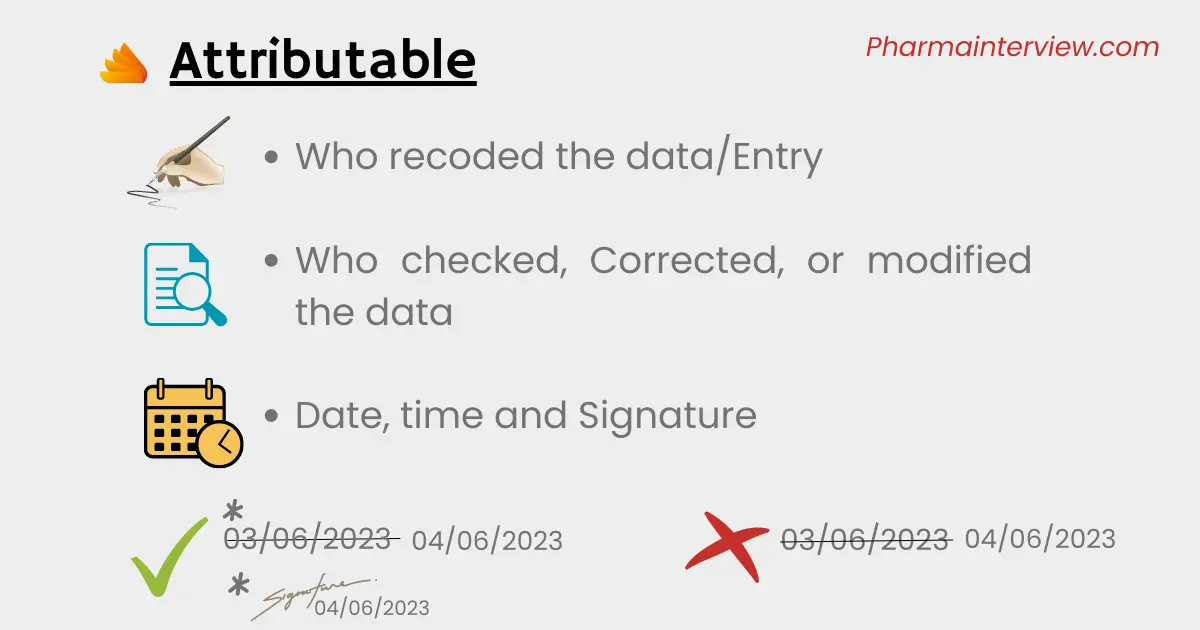
A: Attributable –This principle ensures that data is linked to its source and allows for traceability. It helps to answer the question of who is responsible for generating or modifying the data.
When recording the documentation, it is important to attribute it to a particular individual. This means including the name of the person responsible for compiling the data, along with the date and time. If any corrections or modifications are made to the document, the name of the person making the changes, as well as the time and date should also be recorded with signature. This practice enables the traceability of the data’s source or origin.
Legible meaning in ALCOA plus
L: Legible – Legibility emphasizes the importance of data being easily readable and understandable. It ensures that information can be comprehend and interpreted without any doubt.
Making sure data is easy to read is more than just being able to understand it clearly, although that’s important when keeping records by hand. With electronic data, it’s usually not a big problem to see words and numbers clearly.
But even with electronic data, it’s still important for it to be easy to read and understand, even many years after it was recorded. This applies to both digitally recorded data and data written down in notebooks.
That’s why it’s important to avoid using fancy or unusual words and phrases. They might be hard to understand in the future, especially if the person who created the data isn’t around anymore to explain it.
It’s best to use clear and simple language throughout an organization, no matter where it is.
One last thing to remember about making data easy to read is that the data collected, created, or updated must be permanent. It should stay available and understandable for a long time.

Contemporaneous meaning in ALCOA plus
C: Contemporaneous – Contemporaneous means that data should be recorded at the time it is generated or observed. It promotes the accuracy and reliability of data by preventing delayed or retrospective entries.
It’s very important to record data whenever something happens or an action is taken. When it comes to electronic data, it’s usually normal to include the time when it was recorded, but there are a few things to consider. For example, we need to make sure that data doesn’t get stuck in a queue and delay the recording of the time. We also need to make sure that the clocks on the systems are accurate and that we record the correct time zones.
Overall, though, recording data as soon as possible is especially important when keeping records manually. The main goal is to avoid creating or updating data at a later time. When we record data after an event or action has happened, mistakes can occur. We might forget certain details, leave out important parts, or record information incorrectly.

Original meaning in ALCOA plus
O: Original – Originality signifies that data should be the first record or a certified true copy of the original information. It emphasizes the preservation of the primary source without any alterations.
It’s better to have original records instead of copies or transcriptions, especially when keeping records manually. For example, if you write information on a scrap of paper with the plan to transfer it later, mistakes can happen.
Instead, the first recording of the data should be the main record, whether it’s on paper or in a digital system. When it comes to digitally recorded data, there should be technical and procedural measures in place to make sure the original recording cannot be changed.
Any analysis, reports, or calculations based on the data should be able to be traced back to the original source.
Also, if there are copies of the original record, they should be officially verified as true copies, and they should be clearly different from the original. It’s important to keep the original version of the data, even if there are copies.

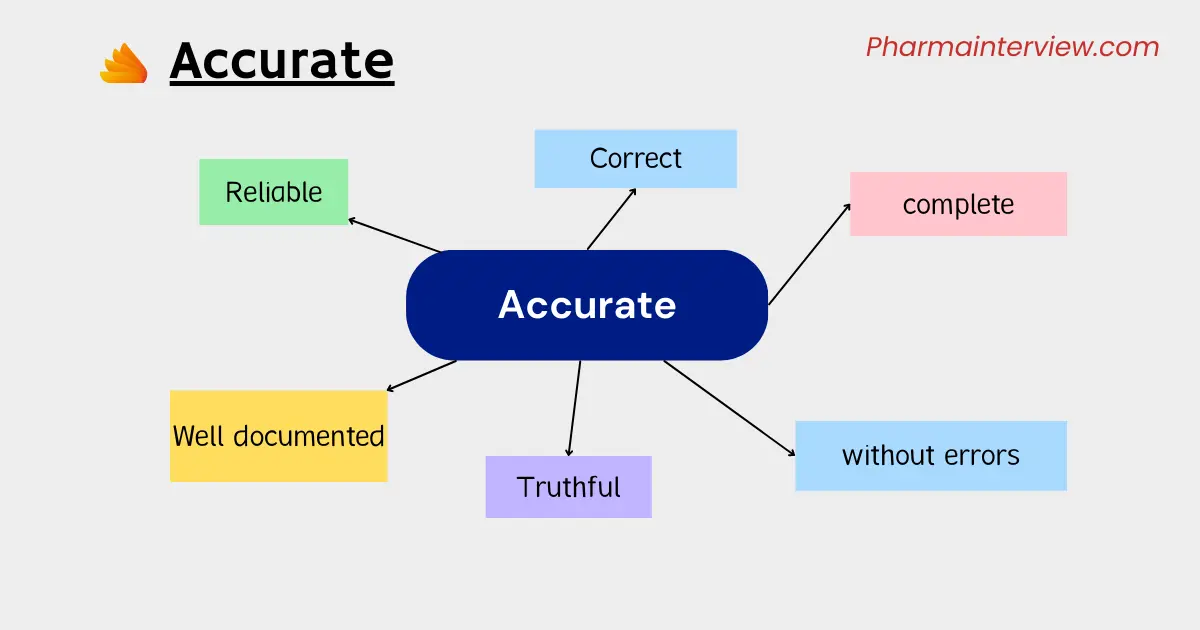
Accurate meaning in ALCOA plus
A: Accurate – Accuracy is a fundamental aspect of data integrity. It requires data to be correct, free from errors, and consistent with the observed or intended results.
All records should show exactly what really happened, and they should be free of mistakes. We shouldn’t edit the original information in a way that makes us lose that information.
If we need to make changes, we have to document them in a way that lets us go back to the original information. We shouldn’t remove or delete anything important.
When we record data electronically, the system should have built-in checks to make sure it’s accurate, and there should be controls to verify the information. We should also regularly check and adjust our measuring tools to make sure they’re accurate.
Additionally, the “plus” in ALCOA+ introduces further principles that complement the original ALCOA framework:
Complete meaning in ALCOA plus
C: Complete – Completeness ensures that all necessary information is included and nothing is omitted. It prevents partial or fragmented data that could lead to misinterpretation.
Every piece of data that is recorded should have a Audit trail that shows if anything has been deleted or lost. This doesn’t only include the main data, but also information about when it was recorded, retested, analyzed, and so on. There should also be a trail that keeps track of any changes made to the data.
Consistent meaning in ALCOA plus
C: Consistent – Consistency requires data to be uniform and coherent across different records and sources. It ensures that there are no conflicting or contradictory pieces of information.
Consistency means keeping data in chronological order, with a date and time stamp that follows a logical sequence. If any changes are made to the original data, they should be marked with a timestamp.
Enduring meaning in ALCOA plus
E: Enduring – Enduring signifies that data should be preserved and maintained throughout its designated retention period. It ensures data integrity over time, allowing for proper historical reference and analysis.
ALCOA+ focuses on making sure data is available for a long time, even decades after it’s recorded. This means taking steps to ensure data durability, especially for digitally recorded data. It involves having strong data backup systems, disaster recovery plans, uninterruptible power supplies, and considering cybersecurity.
Available meaning in ALCOA plus
A: Available – Availability emphasizes that data should be accessible when needed. It should be stored securely and retrievable for authorized personnel to support decision-making and regulatory compliance.
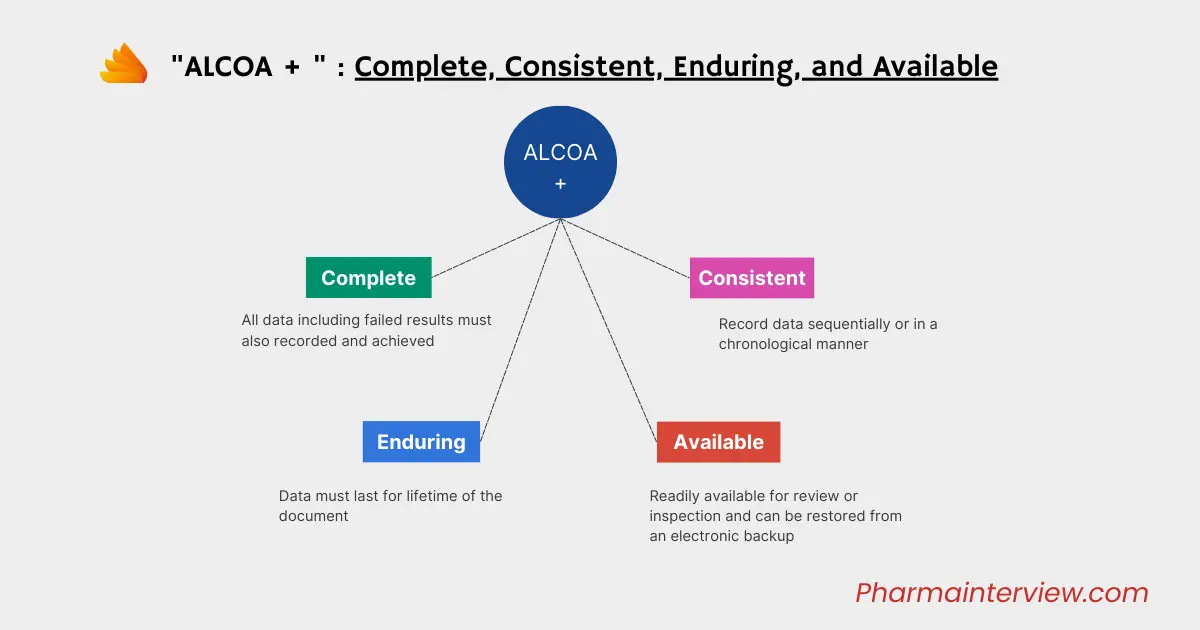
Data needs to not just exist, but also be easy to find and access. This means having storage systems that allow searching, proper indexing, and clear labels. The most effective way to do this is usually by recording data electronically. The data must be readable at any time during the retention period, whether for audits, reviews, inspections, or other purposes.
By incorporating these ALCOA+ principles into data management systems, businesses can uphold the accuracy, reliability, and compliance of their collected data.
Visitors are also reading :
- Top 10 most effective root cause analysis investigation tools
- What is root cause analysis ?
- Change Control Management in pharmaceutical industry
- Handling of deviation in pharma industry
- Dissolution test for solid dosage forms
ALCOA Plus Principles for Data Integrity- FAQ
What is data integrity in pharma ?
Data integrity in pharma refers to ensuring that the data generated, recorded, and reported in the pharmaceutical industry is accurate, complete, consistent, and reliable, in order to comply with regulations, ensure patient safety, maintain product quality, and support scientific research and development.
What is the full form of ALCOA ++?
ALCOA ++ stands for :
A – Attributable
L – Legible
C – Contemporaneous
O – Original
A – Accurate
++ – Additional principles include Consistent, Complete, Enduring, and Available.
What is ALCOA ++ GDP?
ALCOA++ GDP refers to applying the ALCOA++ principles of data integrity specifically to the Good Distribution Practice (GDP) guidelines in the pharmaceutical industry.
What does ALCOA+ apply to?
ALCOA+ applies to the principles of data integrity in regulated industries, including the pharmaceutical industry.
What is the difference between ALCOA and ALCOA+ ?
ALCOA focuses on five key principles of data integrity, while ALCOA+ expands on those principles by adding additional criteria such as consistency, completeness, enduring, and availability.
What is ALCOA- C used for ?
In clinical research, the acronym ALCOA-C is sometimes used. It stands for Attributable, Legible, Contemporaneous, Original, Accurate, and Complete. The purpose of ALCOA-C is to maintain high-quality source documents throughout the entire study.
Is data integrity part of GMP?
Yes, data integrity is an essential component of Good Manufacturing Practices (GMP) in the pharmaceutical industry.
Who created ALCOA+?
The ALCOA+ framework was developed by the pharmaceutical industry and regulatory authorities to enhance the principles of data integrity established by the ALCOA acronym.
Why is ALCOA important?
ALCOA is important because it provides a framework for ensuring data integrity, accuracy, and reliability, which is crucial for maintaining product quality, regulatory compliance, and patient safety in industries such as pharmaceuticals.
Nice to read and understand easily