CAPA (Corrective and Preventive Actions) compliance is a crucial process for pharmaceutical industry to address deviation, event, improve quality, and prevent future issues. In this article, we will delve into the key steps involved in implementing CAPA and discuss best practices for each stage.
Corrective Action: Action required to correct and prevent a re-occurrence for something that happened yesterday, Preventive Action: Action required to prevent an occurrence of something that may happen tomorrow.

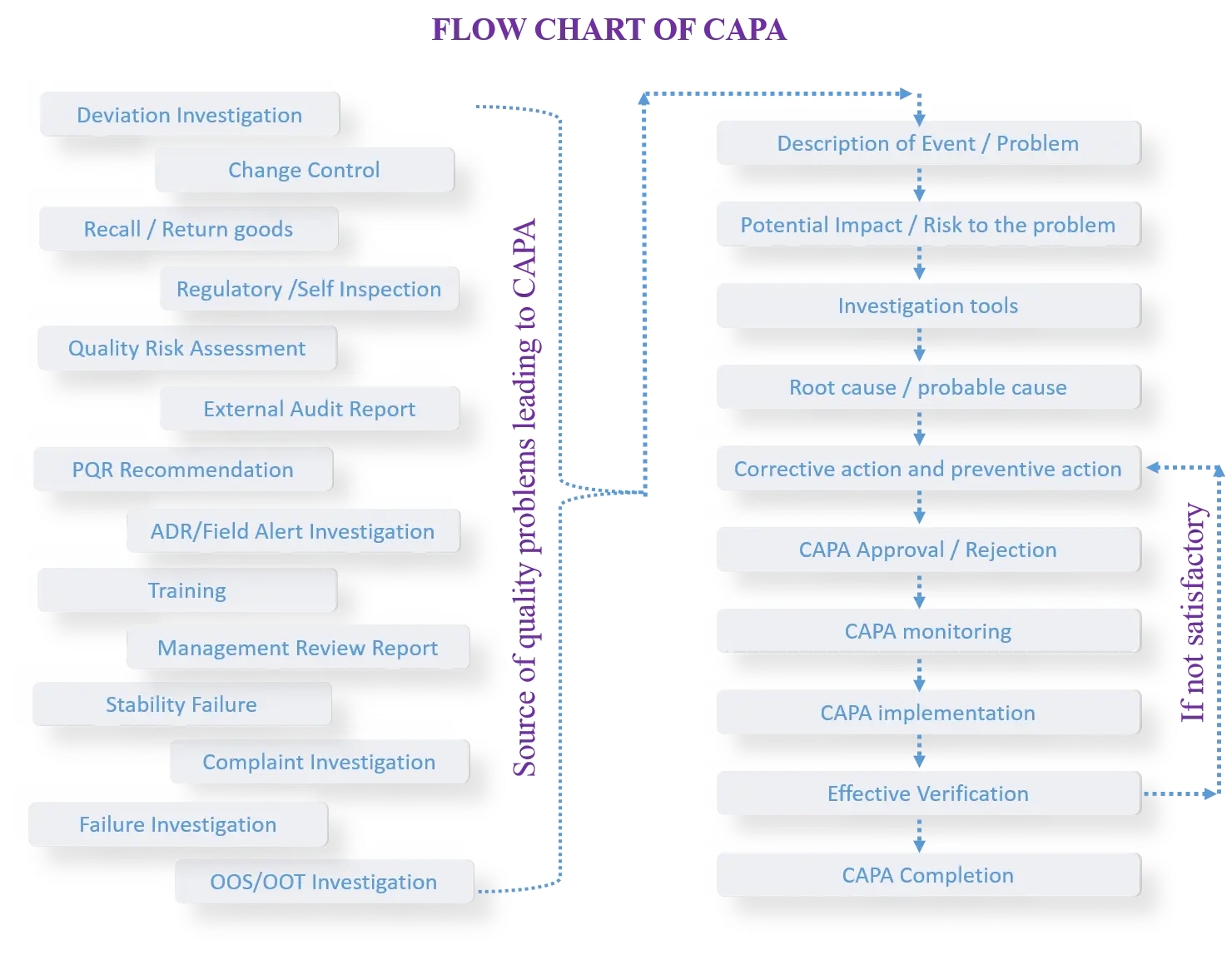
Effective CAPA (Corrective and Preventive Actions) involves the following 8 steps:
Identify and Report the Deviation or Event
Identify the issue or problem that needs to be addressed. This could be a customer complaint, a quality deviation, or any other non-conformance.
The first step in implementing CAPA is identifying the problem or issue that needs to be addressed. This could be a market complaint, a quality deviation, an audit observation, or any other non-conformance. There should be a SOP for handling the deviation.
The source of quality problems leading to CAPA could be following, but not limited to:
- Change Control and its trends
- Deviations/Incidents and its Trend
- Market Complaints and its Trend
- Out of Specification Results and its Trend
- Stability Results, Out of Trends
- Product Recalls and/or Field Actions, such as Field Alert Reports
- Material / Batch Failure, Self Inspection/Audits
- Regulatory Audit and Commitments (Query/deficiency received post submission to any regulatory agency)
- Audit by Contract Giver
- Technology Transfer Document
- PQR, Environment and its Safety
- Quality Control Stability Reports
- Return Goods, Other Non Conformances
- Risk Assessment
- Recommendation of Executed Validation
- Adverse Reaction Reported, Supplier Non Conformance
- Process Control Data Review
- Instrument/Equipment Service Data Review
- Calibration Review, Management Review Results
- Scrap, Yield or Rework Data
Organizations should establish clear channels of communication for deviation reporting and encourage employees to report deviation promptly. This can be achieved through tools such as a centralized quality management system, electronic reporting software like trackwise, and regular quality training.
By having a well-defined process for deviation identification, organizations can ensure that all event are reported and can initiate the CAPA process promptly.
Investigate the Root Cause
Once a deviation has been reported, the next step is to conduct a thorough investigation to determine the root cause. The investigation process should be systematic and involve gathering relevant data, analyzing facts, and involving cross-functional teams if necessary.
Various problem-solving techniques can be utilized during the investigation phase. These include but are not limited to:
- Root Cause Analysis (RCA): RCA helps identify the underlying cause of the problem by systematically tracing it back to its origin. Techniques such as the 5 Whys, fishbone diagrams (Ishikawa diagrams), or fault-tree analysis can be employed.
- Data Analysis: Analyzing data, such as process performance metrics, customer feedback, or quality records, can provide valuable insights into potential causes.
- Process Mapping: Visualizing the process flow and identifying process steps or interactions that may contribute to the problem.
- Brainstorming: Engaging a diverse group of individuals to generate ideas and hypotheses about the potential causes.
The investigation should be documented thoroughly, capturing all relevant findings and data. This documentation will serve as a reference point for subsequent steps in the CAPA process.
Develop Corrective Actions
Based on the findings of the investigation, the next step is to develop appropriate corrective actions. Corrective actions are measures taken to address the root cause of the problem and eliminate its recurrence. It is crucial to consider the following principles while developing corrective actions:
- Effectiveness: The corrective actions should be effective in addressing the root cause and resolving the identified problem. They should directly target the underlying issue to prevent its recurrence.
- Feasibility: The proposed corrective actions should be feasible to implement within the organization’s resources, capabilities, and constraints. Consider factors such as cost, time, and availability of expertise or technology.
- Risk Assessment: Evaluate the potential risks associated with implementing the corrective actions. Ensure that the proposed actions do not introduce new risks or adverse effects on other processes or products.
- Preventive Approach: Whenever possible, adopt a preventive approach by implementing actions that not only address the current problem but also mitigate the risk of similar issues occurring in the future.
It is advisable to involve relevant stakeholders and subject matter experts during the development of corrective actions. Their input can provide valuable insights and ensure the feasibility and effectiveness of the proposed measures.
Implement Corrective Actions
Once the corrective actions have been developed, the next step is their implementation. This stage involves executing the planned actions within the organization. Some best practices for implementing corrective actions include:
- Clearly Communicate: Ensure that all stakeholders are aware of the planned actions, their objectives, and the expected outcomes. Transparent communication helps foster understanding, collaboration, and support throughout the implementation process.
- Assign Responsibilities: Clearly define roles and responsibilities for implementing the corrective actions. Assign dedicated individuals or teams to oversee the execution, monitoring, and coordination of the actions.
- Establish Timelines: Develop a timeline or project plan that outlines the key milestones and deadlines for implementing the corrective actions. This helps create accountability and ensures timely completion.
- Monitor Progress: Continuously monitor the progress of corrective action implementation. Regularly review the status, identify any challenges or deviations, and take necessary corrective measures to keep the process on track.
- Training and Awareness: If the corrective actions involve changes in processes, procedures, or employee behavior, provide appropriate training and awareness sessions. Ensure that all individuals involved understand the changes and are equipped to implement them effectively.
Validate the Effectiveness
Validating the effectiveness of the implemented corrective actions is a critical step in the CAPA process. It involves evaluating whether the actions have successfully resolved the problem without creating new ones. The following practices can help in the validation process:
- Data Analysis: Analyze relevant data to assess whether the problem has been resolved. Compare performance metrics before and after the implementation of corrective actions to gauge improvement.
- Performance Testing: Conduct tests or experiments to verify the effectiveness of the implemented actions. This could involve process trials, product testing, or simulation exercises to evaluate the impact.
- Customer Feedback: Gather feedback from customers or end-users to determine their satisfaction levels and whether the problem has been adequately addressed.
- Process Audits: Conduct internal audits or inspections to verify that the corrective actions have been implemented as planned and are being followed consistently.
The validation process should be documented, capturing the results, conclusions, and any lessons learned. This documentation serves as evidence that the problem has been resolved and can be referenced in the future.
Implement Preventive Actions
In addition to corrective actions, organizations should also focus on implementing preventive actions. Preventive actions are measures taken to proactively identify and mitigate potential problems before they occur. This helps prevent future issues and promotes continuous improvement. Some best practices for implementing preventive actions include:
- Risk Assessment: Identify potential risks and vulnerabilities in processes, products, or systems. Conduct risk assessments to prioritize and address these risks through preventive measures.
- Process Optimization: Continuously evaluate and improve processes to minimize the likelihood of errors or deviations. Implement process controls, standard operating procedures, or automation to enhance efficiency and reduce variability.
- Training and Education: Invest in training programs to enhance employee skills and knowledge. Ensure that employees are equipped to perform their tasks effectively and understand the importance of quality and preventive measures.
- Performance Monitoring: Establish monitoring systems to track key performance indicators and identify early warning signs of potential issues. Regularly analyze data and trends to proactively address emerging risks.
Document and Track
Throughout the CAPA process, it is essential to maintain thorough documentation and records. Document the problem identification, investigation findings, developed actions, implementation details, validation results, and any associated communication. Proper record-keeping ensures transparency, accountability, and facilitates future reference and analysis. Use a centralized document management system or quality management software to organize and store CAPA-related documents efficiently.
Continuous Improvement
Implementing CAPA is an iterative process that requires continuous improvement and review. Regularly evaluate the effectiveness of implemented actions and assess the overall CAPA process. Analyze data, solicit feedback, and conduct periodic audits to identify areas for improvement. Consider engaging in benchmarking activities or seeking external expertise to gain new perspectives and best practices. By embracing a culture of continuous improvement, organizations can enhance their quality management systems, minimize risks, and drive excellence.
Conclusion
In conclusion, the implementation of CAPA is a process for solving problems, improving quality, and preventing future issues. By following the basic steps outlined above and adhering to best practices, organizations can implement effective CAPA programs that drive continuous improvement and ensure customer satisfaction.
People also read :
CAPA Compliance – FAQ
What is CAPA in GMP?
CAPA in GMP stands for Corrective and Preventive Actions. It is a quality management system used in pharmaceutical manufacturing to identify and address deviation, ensuring product quality and compliance.
What is RCA and CAPA?
RCA stands for Root Cause Analysis, which is a method used to identify the underlying cause or causes of a deviation or event. CAPA stands for Corrective and Preventive Actions, which are measures taken to address and resolve identified deviation and prevent their recurrence.
What is CAPA life cycle?
The CAPA (Corrective and Preventive Actions) life cycle process include following steps for corrective and preventive actions in a systematic manner.
1. Identification
2. investigation
3. implementation
4. validation
it also address problems and continuous quality improvement of product.
What is CAPA risk level?
CAPA risk level refers to the assessment of the potential impact and likelihood of risks associated with the implementation of Corrective and Preventive Actions (CAPA). It helps prioritize and determine the level of attention and resources required to address the identified risks during the CAPA process.
Who implements CAPA?
CAPA is applicable in various industries like pharmaceuticals, manufacturing, healthcare, and other regulated sectors. CAPA implementation required cross-department’s employees involvement at different levels within the organization. CAPA implementation team need to identify, investigate, develop, implement, and validate corrective and preventive actions.
Is CAPA part of ISO 9001?
Yes, CAPA is a part of ISO 9001 (quality management system) standard. CAPA is included in the ISO 9001:2015 standard in “Control of Nonconforming Outputs” heading. it describe the need for organizations to identify nonconformities and also to take appropriate corrective and preventive actions to prevent their recurrence.
all information is good but we don’t have facility to copy this content.