21 CFR guidelines for pharmaceuticals food and drugs industry is widely practiced and has great importance in digital documentation. 21 CFR is well known in pharmaceuticals for the 21 CFR Part 11 – electronic signature, 21 CFR Part 210, 21 CFR Part 211 and 21 CFR Part.

How many titles are there in the Code of Federal Regulations (CFR) ?
In the United States, the Code of Federal Regulations (CFR) is a collection of rules made by the government. These rules are made by different departments and agencies and cover different areas. The Code of Federal Regulations (CFR) is divided into 50 titles that represent broad areas subject to federal regulation and out of 50 titles of the Code of Federal Regulations (CFR), Title 21 CFR is about Food and Drugs.
Read : Full List of 50 titles of The Code of Federal Regulations (CFR)
Title 21 CFR (Code of Federal Regulations) – Food and Drugs
The 21 CFR and its guideline are very important in the pharmaceutical industry. It’s like a rule book for food and drugs in the United States. There are three important governing bodies are responsible for compliance of 21 CFR guideline : The FDA (Food and Drug Administration), DEA (Drug Enforcement Agency) and ONDCP (Office of National Drug Control Policy).
The CFR (Code of Federal Regulations) is a codification of the general and permanent rules published in the Federal Register by the Executive departments and agencies of the Federal Government. Title 21 of the CFR is reserved for rules of the Food and Drug Administration. Each title (or volume) of the CFR is revised once each calendar year. A revised Title 21 is issued on approximately April 1st of each year.
How many parts are in 21 CFR?
FDA’s portion of the CFR is in Title 21, which interprets the Federal Food, Drug and Cosmetic Act and related statutes, including the Public Health Service Act. The 21 CFR guidelines for pharmaceuticals – Food and Drugs: contains 3 chapters – I, II, III and parts – 1 to 1499. The pharmaceutical or drug quality-related regulations appear in several parts of Title 21, including sections in parts 1-99, 200-299, 300-499, 600-799, and 800-1299.
21 CFR guidelines for pharmaceuticals
21 CFR guidelines for pharmaceuticals is divided into three chapters, each specializing in codes for these organizations.
- Chapter 1 : Food and Drug administration
- Chapter 2 : Drug Enforcement Administration
- Chapter 3 : Office of National Drug Control Policy
Chapter 1 : FDA (Food and drug administration) department of Health and Human services, Parts: 1 – 1299
Subchapter A – General (Parts: 1 – 99)
Subchapter B – Food for Human Consumption (Parts: 100 – 199)
Subchapter C – Drugs: General (Parts: 200 – 299)
Subchapter D – Drugs for Human Use (Parts: 300 – 499)
Subchapter E – Animal Drugs, Feeds, and Related Products (Parts: 500 – 599)
Subchapter F – Biologics (Parts: 600 – 680)
Subchapter G – Cosmetics (Parts: 700 – 799)
Subchapter H – Medical Devices (Parts: 800 – 898)
Subchapter I – Mammography Quality Standards Act (Parts: 900)
Subchapter J – Radiological Health (Parts: 1000 – 1050)
Subchapter K – Tobacco Products (Parts: 1100 – 1150)
Subchapter L – Regulations Under Certain Other Acts Administered by the FDA (Parts: 1210 – 1299)
Chapter 1 of 21 CFR guidelines for pharmaceuticals is specifically meant for the Food and Drug Administration (FDA). It has been derived from the Federal food, drug and cosmetic act. This chapter has many sections dealing with various guidelines. Some of the most prominent ones are as follows:
- 21 CFR Parts 11: Electronic Records; Electronic Signatures. it applies to records in electronic form that are created, modified, maintained, archived, retrieved, or transmitted under any records requirements set as per FDA regulations.
- 21 CFR Parts 50: Protection of Human Subjects. Guideline to ensure protection of human subjects in clinical trials.
- 21 CFR Parts 54: Financial Disclosure by Clinical Investigators. Full information of financial records by clinical investigators.
- 21 CFR Parts 56: Clinical trials Guidelines for institutional review boards.
- 21 CFR Parts 58: Good Laboratory Practices.
- 21 CFR Parts 101: Food Labeling.
- 21 CFR Parts 202: Prescription Drug Advertising
- 21 CFR Parts 205:Guidelines for State Licensing of Wholesale Prescription Drug Distributors.
- 21 CFR Parts 210: Current Good Manufacturing Practice in Manufacturing, Processing, Packing, or Holding of Drugs; General
- 21 CFR Parts 211: Current Good Manufacturing Practice for Finished Pharmaceuticals
- 21 CFR Parts 500 to 21 CFR Parts 599 : Guidelines for veterinary drugs and medications application for animals and practices related to the veterinary sciences.
- 21 CFR Parts 600 to 21 CFR Parts 680 : Guidelines covers biological substances like blood, lymph and vaccines.
- 21 CFR Parts 700 series describe requirements for specific cosmetic product like labeling, research, registration procure and Ingredient Composition.
- 21 CFR Parts 820 : Quality System Regulation for medical devices.
- 21 CFR Parts 800 series is applicable for medical devices, their Labeling, hazard warnings, premarket approval requirement, classification and procedures for performance standards development of medical devices.
- 21 CFR Parts 900 series regulates the Quality standards necessary for devices used for mammography.
- 21 CFR Parts 1000 series enforces requirements of radiological devices for example x-ray machines.
- 21 CFR Parts 1100 series deals with tobacco products such as e-cigarettes, pipe tobacco, etc.
- 21 CFR Parts 1200 series deals with products which are not included in food, drug and cosmetic act for example pasteurization of milk, import and export of turtles as pets, etc.
Chapter 2 : DEA (Drug Enforcement Administration) Department of Justice, Parts: 1300 – 1399
Part 1300 – Definitions
Part 1301 – Registration of Manufacturers, Distributors, and Dispensers of Controlled Substances
Part 1302 – Labeling and Packaging Requirements for Controlled Substances
Part 1303 – Quotas
Part 1304 – Records and Reports of Registrants
Part 1305 – Orders for Schedule I and II Controlled Substances
Part 1306 – Prescriptions
Part 1307 – Miscellaneous
Part 1308 – Schedules of Controlled Substances
Part 1309 – Registration of Manufacturers, Distributors, Importers and Exporters of List I Chemicals
Part 1310 – Records & Reports of Listed Chemicals and Certain Machines
Part 1311 – Requirements for Electronic Orders and Prescriptions
Part 1312 – Importation and Exportation of Controlled Substances
Part 1313 – Importation and Exportation of List I and List II Chemicals
Part 1314 – Retail Sale of Scheduled Listed Chemical Products
Part 1315 – Importation and Production Quotas for Ephedrine, Pseudoephedrine, and Phenylpropanolamine
Part 1316 – Administrative Functions, Practices, and Procedures
Part 1317 – Disposal
Part 1318 – Controls to Satisfy the Requirements of the Act Applicable to the Manufacturing of Marihuana
Part 1321 – DEA Mailing Addresses
Part 1322 -1399 – [Reserved]
Chapter 2 of the 21 CFR guidelines for pharmaceuticals addresses the regulations surrounding the marketing, sale, and utilization of controlled substances and scheduled drugs. Within this chapter, there is a comprehensive listing of the specific drugs and substances that necessitate control measures, along with the stipulation that special documentation is necessary for their purchase and sale.
Chapter 3 : ONDCP (Office of National Drug Control Policy), Parts: 1400 – 1499
Part 1400 – [Reserved]
Part 1401 – Public Availability of Information
Part 1402 – Mandatory Declassification Review
Parts 1403-1499 – [Reserved]
Chapter 3 of the 21 CFR guidelines for pharmaceuticals primarily focuses on the regulations governing the establishment of drug-free environments within government workplaces. This chapter encompasses guidelines regarding prohibited substances, as well as the appropriate tests and procedures that employees in these settings must undergo. Additionally, it outlines the frequency at which these tests should be conducted in accordance with the established rules.
What is 21 CFR Part 11 ?
21 CFR Part 11 – Electronic records and Electronic signature.
Subpart A — General Provisions
Scope
- The regulations in 21 CFR Part 11 set forth the criteria under which the agency considers electronic records, electronic signatures, and handwritten signatures executed to electronic records to be trustworthy, reliable, and generally equivalent to paper records and handwritten signatures executed on paper.
- 21 CFR Part 11 applies to records in electronic form that are created, modified, maintained, archived, retrieved, or transmitted, under any records requirements set forth in agency regulations. This part also applies to electronic records submitted to the agency under requirements of the Federal Food, Drug, and Cosmetic Act and the Public Health Service Act.
- 21 CFR Part 11 guideline consider the electronic signatures to be equivalent to full handwritten signatures, initials, and other general signings.
- Electronic records that meet the requirements of this part may be used in lieu of paper records.
- Computer systems (including hardware and software), controls, and attendant documentation maintained under this part.
Implementation
Electronic records and signatures required to be maintained but not required submitted to the FDA, moreover persons may use electronic records in the place of paper records or electronic signatures in the place of traditional signatures.
Electronic records and signatures records can be submitted to the FDA, if (1) The requirements of 21 CFR Part 11 guideline are met; and (2) The document or parts of a document to be submitted have been identified in public docket No. 92S–0251 as being the type of submission the agency accepts in electronic form.
Definitions
Biometrics means a method of verifying an individual’s identity based on measurement of the individual’s physical feature(s) or repeatable action(s) where those features and/or actions are both unique to that individual and measurable.
Digital signature means an electronic signature based upon cryptographic methods of originator authentication, computed by using a set of rules and a set of parameters such that the identity of the signer and the integrity of the data can be verified.
Electronic record means any combination of text, graphics, data, audio, pictorial, or other information representation in digital form that is created, modified, maintained, archived, retrieved, or distributed by a computer system.
Electronic signature means a computer data compilation of any symbol or series of symbols executed, adopted, or authorized by an individual to be the legally binding equivalent of the individual’s handwritten signature.
Handwritten signature means the scripted name or legal mark of an individual handwritten by that individual and executed or adopted with the present intention to authenticate a writing in a permanent form. The act of signing with a writing or marking instrument such as a pen or stylus is preserved. The scripted name or legal mark, while conventionally applied to paper, may also be applied to other devices that capture the name or mark.
Subpart B — Electronic Records
Controls for closed systems
Persons who use closed systems to create, modify, maintain, or transmit electronic records shall employ procedures and controls designed to ensure the authenticity, integrity, and the confidentiality of electronic records, and to ensure that the signer cannot readily repudiate the signed record as not genuine. Such procedures and controls shall include the following:
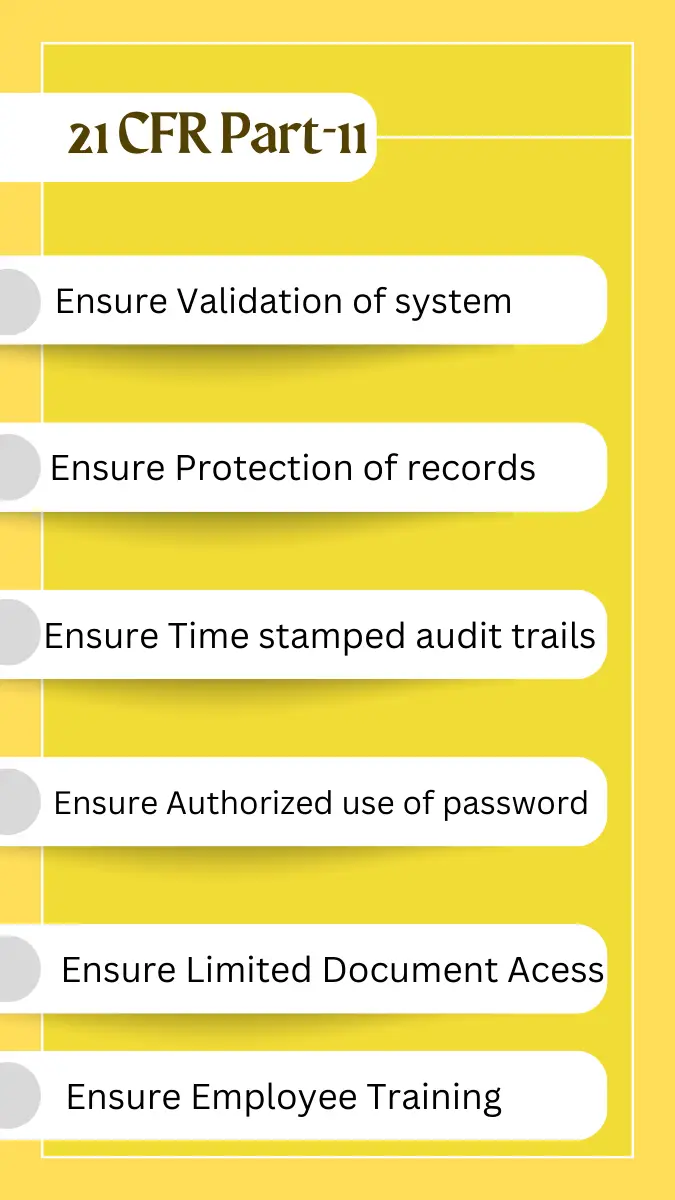
(a) Validation of systems to ensure accuracy, reliability, consistent intended performance, and the ability to discern invalid or altered records.
(b) The ability to generate accurate and complete copies of records in both human readable and electronic form suitable for inspection, review, and copying by the FDA. Persons should contact the FDA if there are any questions regarding the ability of the agency to perform such review and copying of the electronic records.
(c) Protection of records to enable their accurate and ready retrieval throughout the records retention period.
(d) Limiting system access to authorized individuals.
(e) Use of secure, computer-generated, time-stamped audit trails to independently record the date and time of operator entries and actions that create, modify, or delete electronic records. Record changes shall not obscure previously recorded information. Such audit trail documentation shall be retained for a period at least as long as that required for the subject electronic records and shall be available for agency review and copying.
(f) Use of operational system checks to enforce permitted sequencing of steps and events, as appropriate.
(g) Use of authority checks to ensure that only authorized individuals can use the system, electronically sign a record, access the operation or computer system input or output device, alter a record, or perform the operation at hand.
(h) Use of device (e.g., terminal) checks to determine, as appropriate, the validity of the source of data input or operational instruction.
(i) Determination that persons who develop, maintain, or use electronic record/electronic signature systems have the education, training, and experience to perform their assigned tasks.
(j) The establishment of, and adherence to, written policies that hold individuals accountable and responsible for actions initiated under their electronic signatures, in order to deter record and signature falsification.
(k) Use of appropriate controls over systems documentation including:
(1) Adequate controls over the distribution of, access to, and use of documentation for system operation and maintenance.
(2) Revision and change control procedures to maintain an audit trail that documents time-sequenced development and modification of systems documentation.
Controls for open systems
Persons who use open systems to create, modify, maintain, or transmit electronic records shall employ procedures and controls designed to ensure the authenticity, integrity, and, as appropriate, the confidentiality of electronic records from the point of their creation to the point of their receipt. Such procedures and controls shall include those identified in controls for closed systems, as appropriate, and additional measures such as document encryption and use of appropriate digital signature standards to ensure, as necessary under the circumstances, record authenticity, integrity, and confidentiality.
Signature manifestations
Signed electronic records shall contain information associated with the signing that clearly indicates the printed name of the signer, the date and time when the signature was executed, the meaning (such as review, approval, responsibility, or authorship) associated with the signature and part of any human readable form of the electronic record (such as electronic display or printout).
Signature/record linking
Electronic signatures and handwritten signatures executed to electronic records shall be linked to their respective electronic records to ensure that the signatures cannot be excised, copied, or otherwise transferred to falsify an electronic record by ordinary means.
Subpart C— Electronic Signatures
General requirements
- Each electronic signature shall be unique to one individual and shall not be reused by, or reassigned to, anyone else.
- Before an organization establishes, assigns, certifies, or otherwise sanctions an individual’s electronic signature, or any element of such electronic signature, the organization shall verify the identity of the individual.
- Persons using electronic signatures shall certify to the agency that the electronic signatures in their system are intended to be the legally binding equivalent of traditional handwritten signatures.
- The certification shall be signed with a traditional handwritten signature and submitted in electronic or paper form. Information on where to submit the certification can be found on FDA’s web page on Letters of Non-Repudiation Agreement.
- Persons using electronic signatures shall, upon agency request, provide additional certification or testimony that a specific electronic signature is the legally binding equivalent of the signer’s handwritten signature.
Electronic signature components and controls
(a) Electronic signatures that are not based upon biometrics shall employ at least two distinct identification components such as an identification code and password and shall be used only by their genuine owners and shall be administered and executed to ensure that attempted use of an individual’s electronic signature by anyone other than its genuine owner requires collaboration of two or more individuals.
(b) Electronic signatures based upon biometrics shall be designed to ensure that they cannot be used by anyone other than their genuine owners.
Controls for identification codes/passwords
Persons who use electronic signatures based upon use of identification codes in combination with passwords shall employ controls to ensure their security and integrity. Such controls shall include:
- Maintaining the uniqueness of each combined identification code and password, such that no two individuals have the same combination of identification code and password.
- Ensuring that identification code and password issuances are periodically checked, recalled, or revised (e.g., to cover such events as password aging).
- Following loss management procedures to electronically deauthorize lost, stolen, missing, or otherwise potentially compromised tokens, cards, and other devices that bear or generate identification code or password information, and to issue temporary or permanent replacements using suitable, rigorous controls.
- Use of transaction safeguards to prevent unauthorized use of passwords and/or identification codes, and to detect and report in an immediate and urgent manner any attempts at their unauthorized use to the system security unit, and, as appropriate, to organizational management.
- Initial and periodic testing of devices, such as tokens or cards, that bear or generate identification code or password information to ensure that they function properly and have not been altered in an unauthorized manner.
What is 21 CFR part 210 and 211 ?
In 1962, the US government told the FDA to make sure that all medicines are manufactured using good manufacturing practices (GMP) which should be compliant to FDA 21 CFR Part 210 and 21 CFR 211. This was because there were worries about some medicines being made in a bad way, like when a medicine called thalidomide caused birth defects. The 1962 Drug Amendments brought cGMP, modern quality assurance and control control guideline to drug manufacturing to make sure medicines are safe and high quality.
Read : 21 CFR Part 210 – cGMP in Manufacturing, Processing, Packing, or Holding of Drugs; General
Read : 21 CFR Part 211 – cGMP for Finished Pharmaceuticals
Also Read : ALCOA + 9 Principles for Data Integrity
What is 21 CFR in pharma?
Title 21 of the CFR is reserved for rules of the Food and Drug Administration
What does CFR stand for?
The Code of Federal Regulations (CFR) is the codification of the general and permanent rules published in the Federal Register by the departments and agencies of the Federal Government.
Why 21 CFR is required?
The objective behind this regulation is to ensure that the data and information gathered and recorded throughout the product manufacturing process are accurate, traceable, verifiable, suitable for their intended purpose, and safeguarded against loss or unauthorized use.
How many parts are there in CFR?
The CFR (Code of Federal Regulations) is divided into 50 titles that represent broad areas subject to Federal regulation
What is 21 CFR Part 7?
21 CFR 7 provides guidance so that responsible firms may conduct an effective recall. A recall is an alternative to an FDA-initiated court action for removing or correcting violative products that have been distributed.
What is the difference between 21 CFR Part 210 and 211?
Where Part 210 covers manufacturing, facilities and controls, Part 211 covers additional areas for finished drug products, such as labeling, production processes, equipment management and personnel.
What is 21 CFR Part 11 criteria?
21 CFR 11 requires that closed computer systems must have a collection of technological and procedural controls to protect data within the system. Open computer systems must also include controls to ensure that all records are authentic, incorruptible, and (where applicable) confidential.
Very important guideline 21 CFR
If possible please send me all 21cfr groups and clause available in pharmaceutical and food during 21-CFR