ResMed Ltd. Recalls Continuous Positive Airway Pressure (CPAP) Masks with Magnets due to Possible Magnetic Interference with Certain Medical Devices.
ResMed has initiated a recall for specific models of its continuous positive airway pressure (CPAP) masks, namely AirFit and AirTouch, in response to potential magnetic interference with certain medical devices and implants. The FDA has highlighted concerns that such interference may disrupt the normal function or positioning of these devices, posing a risk of serious harm or even death.
FDA three classes of product recall
The FDA uses three classes of recall to indicate the relative degree of health hazard associated with a recalled product:
- Class I: These recalls involve situations where there is a reasonable probability that using or being exposed to the product will cause serious adverse health consequences or death. Examples include products with manufacturing defects that could lead to device failure or contamination, or drugs with incorrect active ingredients or dosages.
- Class II: This class covers products where use or exposure may cause temporary or medically reversible adverse health consequences, or where the probability of serious adverse health consequences is remote. This might include products with labeling errors or minor contamination issues.
- Class III: These are products where use or exposure is not likely to cause adverse health consequences. This could involve issues like cosmetic labeling errors or minor packaging deficiencies.
ResMed’s CPAP Masks with Magnets | Product Recall details:
- Product Names : AirFit and AirTouch masks
- Model Numbers: AirFit N10, AirFit F20, AirTouch F20, AirFit N20, AirTouch N20, AirFit F30, AirFit F30i
- Distribution Dates: January 2020 to November 20, 2023
- Devices Recalled in the U.S.: 20,414,357
AirFit and AirTouch masks Device Use
The AirFit and AirTouch masks are non-continuous ventilatory devices, intended to be used by patients weighing more than 66 lbs. who have been prescribed non-invasive positive airway pressure (PAP) therapy such as CPAP or bi-level therapy. The masks are meant for reuse by one person at home or by multiple people in hospitals.
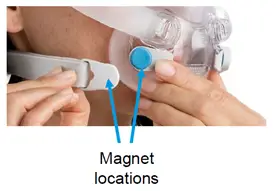
The devices have magnets on the lower headgear straps and frame connections of CPAP masks. These magnets are there to make wearing the mask more comfortable.
Continuous Positive Airway Pressure (CPAP) masks Reason for Recall
ResMed Ltd. has initiated a recall of all its Continuous Positive Airway Pressure (CPAP) masks containing magnets due to potential magnetic interference with specific medical devices. The proximity of the magnets, found within the masks, to certain medical implants and devices within a 2-inch range may disrupt their function or positioning, posing a risk of serious harm or even death.
Although the current label advises maintaining a 2-inch distance from affected medical devices, it does not provide a comprehensive list of specific devices susceptible to the masks’ magnets. In response, ResMed is recalling these masks to update the labels, incorporating additional warnings and information to guide both patients and healthcare professionals on the safe use of masks with magnets. This recall and label update follow a thorough review of potential risks associated with magnetic interference and its impact on medical implants.
The utilization of these affected masks carries the potential for severe adverse health consequences and, in extreme cases, death.
To date, six injuries related to this issue have been reported, while there have been no instances of death reported.
Read more:
FDA Clears First AI-Powered Medical Device to detect Skin Cancers