The Food and Drug Administration (FDA) has approved CASGEVY™ and LYFGENIA™ the first two gene therapies for the treatment of sickle cell disease (SCD).
Key update:
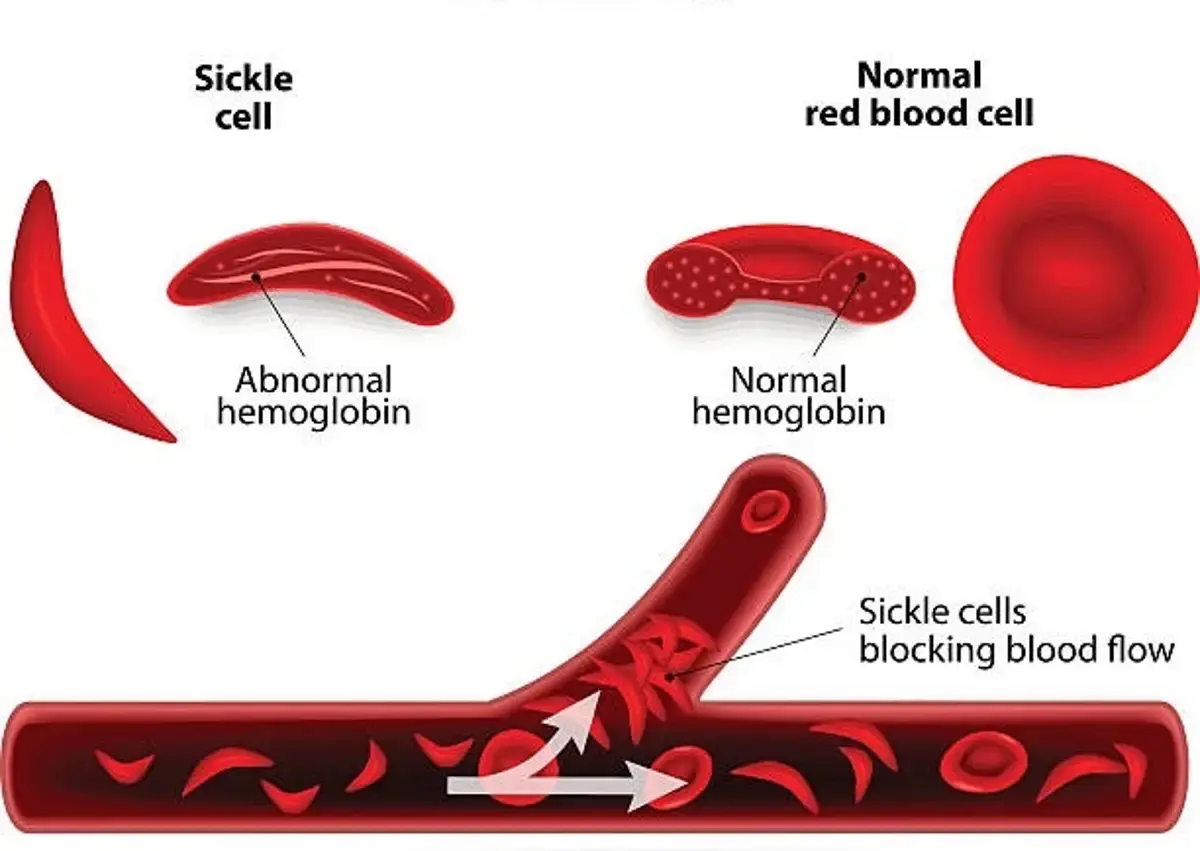
- Sickle cell disease causes red blood cells to take on a sickle-like shape, making them fragile and less able to transport oxygen.
- FDA has approved CASGEVY™ and LYFGENIA™ the first two gene therapies for the treatment of sickle cell disease.
- Two companies, Bluebird Bio and Vertex Pharmaceuticals, just received FDA approval of their gene therapy.
- For the first time, the FDA has approved gene therapies to treat sickle cell disease.
- The treatments change the type of red blood cells made by the body, which eliminates complications of sickle cell disease, such as infections and stroke.
- One treatment uses the CRISPR-Cas9 gene-editing system. The other uses a different method to insert a gene into stem cells.
What is sickle cell disease ?
- Sickle cell disease is a lifelong condition that causes intense pain due to deformed blood cells that can cause blockages in blood vessels. This can also lead to strokes, organ damage, and potentially shortened lives.
- More than 100,000 people live with sickle cell disease in the US, with the most patients being African American.
How gene therapy works ?
LYFGENIA™ gene therapy:
In the case of LYFGENIA, the gene therapy is specifically designed to treat the underlying cause of sickle cell disease by adding a functional gene that enables production of adult hemoglobin that does not form into the crescent shape associated with the disease.
CASGEVY™ gene therapy:
In the case of CASGEVY™ gene therapy, is the first FDA-approved therapy to employ CRISPR-Cas9 genome editing technology, modifying patients’ hematopoietic stem cells to remove the gene that causes sickle cell disease. The modified cells are transplanted back into the patient, where they engraft within the bone marrow. The treatment increases the production of fetal hemoglobin (HbF) to facilitate oxygen delivery and prevent red blood cell (RBC) sickling.
Today marks a significant milestone as the U.S. Food and Drug Administration has given its approval for two groundbreaking treatments—Casgevy and Lyfgenia. These treatments represent the pioneering use of cell-based gene therapies for individuals aged 12 and above who are grappling with sickle cell disease (SCD). Notably, Casgevy, one of the approved therapies, stands out as the first FDA-sanctioned treatment employing a novel genome editing technology, signifying a momentous leap forward in the realm of gene therapy.
Sickle cell disease, a collection of inherited blood disorders, affects around 100,000 individuals in the U.S., with a higher prevalence among African Americans and, to a lesser extent, Hispanic Americans. The core issue in SCD lies in a mutation in hemoglobin, the protein responsible for transporting oxygen to the body’s tissues. This mutation leads to the formation of sickled red blood cells, impeding blood flow and causing severe pain and organ damage, termed vaso-occlusive events (VOEs) or vaso-occlusive crises (VOCs). The recurrence of these crises poses a threat of life-altering disabilities or premature death.
Dr. Nicole Verdun, the director of the Office of Therapeutic Products at the FDA’s Center for Biologics Evaluation and Research, expresses enthusiasm for advancing the field, especially for those whose lives have been severely affected by the disease. She emphasizes the potential of gene therapy to provide more targeted and effective treatments, particularly for rare diseases with limited existing options.
Casgevy, a cell-based gene therapy, has gained approval for treating SCD patients aged 12 and older experiencing recurrent vaso-occlusive crises. Notably, Casgevy is the inaugural FDA-approved therapy utilizing CRISPR/Cas9, a genome editing technology. This involves modifying patients’ hematopoietic stem cells through CRISPR/Cas9 to enhance the production of fetal hemoglobin, preventing the sickling of red blood cells.
Lyfgenia, another cell-based gene therapy, employs a lentiviral vector for genetic modification and is approved for treating SCD patients aged 12 and older with a history of vaso-occlusive events. Lyfgenia modifies patients’ blood stem cells to produce HbAT87Q, a gene-therapy derived hemoglobin that mimics the function of hemoglobin A, reducing the risk of sickling in red blood cells.
Both Casgevy and Lyfgenia utilize patients’ own blood stem cells, which are modified and reintroduced through a one-time, single-dose infusion as part of a hematopoietic stem cell transplant. Prior to treatment, patients’ stem cells are collected, and they undergo myeloablative conditioning to prepare for the transplantation.
Dr. Peter Marks, the director of the FDA’s Center for Biologics Evaluation and Research, highlights the importance of these approvals, emphasizing the use of innovative cell-based gene therapies to address severe diseases and enhance public health. The FDA’s rigorous evaluations of scientific and clinical data support these approvals, showcasing the agency’s commitment to advancing safe and effective treatments.
Detailed data supporting Casgevy and Lyfgenia highlight their safety and effectiveness, with both therapies receiving Priority Review, Orphan Drug, Fast Track, and Regenerative Medicine Advanced Therapy designations. Casgevy is granted to Vertex Pharmaceuticals Inc., while Lyfgenia is granted to Bluebird Bio Inc.