Dissolution test is a Analytical method for measuring the rate of drug release from a Drug product within a specific time period.
Why Dissolution test perform ?
- As per batch release specification
- During stability testing
- At the early stages of the drug development process, in-vitro dissolution testing helps to correlate in-vivo drug-release behavior of developing drug products.

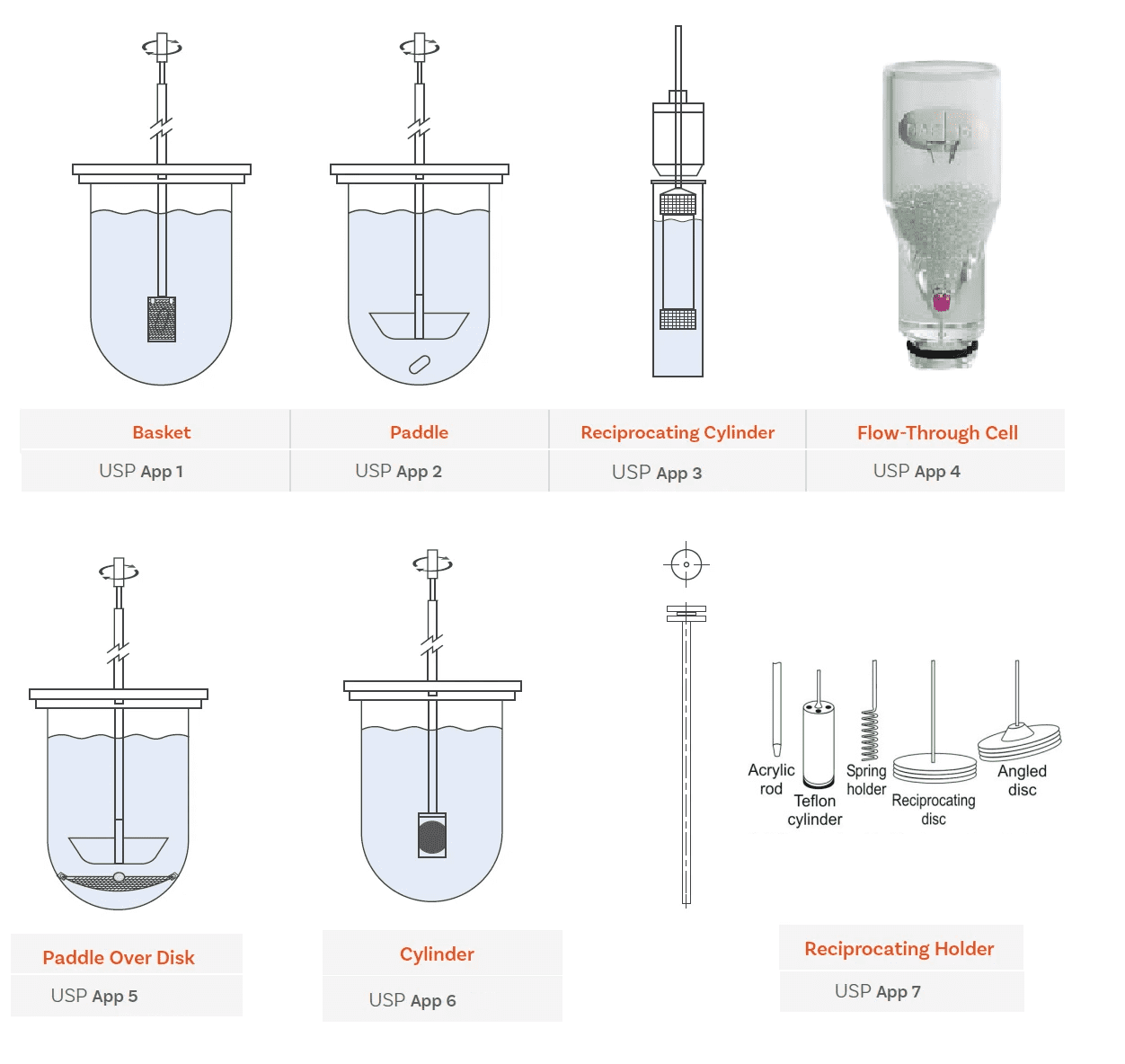
Types of apparatus used for Dissolution test
Immediate, modified and extended release oral solid dosage forms are usually tested in standard dissolution baths with USP Apparatus 2 paddles, For oral dosage forms that are prone to floating, USP Apparatus 1 baskets would generally be required.
- USP Apparatus 1 (Basket Apparatus)
- USP Apparatus 2 (Paddle Apparatus)
- USP Apparatus 3 (Reciprocating Cylinder)
- USP Apparatus 4 (Flow-Through Cell)
- USP Apparatus 5 (Paddle-over-disc)
- USP Apparatus 6 (cylinder)
- USP Apparatus 7 (reciprocating holders)
Dissolution test for Immediate release dosage forms
Procedure Dissolution test
- Use USP Apparatus 1 (Basket Apparatus) or USP Apparatus 2 (Paddle Apparatus) as per product specification
- Place the stated volume of the Dissolution Medium (±1%) in the vessel of the specified apparatus given in the individual monograph.
- Assemble the apparatus
- Equilibrate the Dissolution Medium to 37 ± 0.5° C and remove the thermometer.
- Place 1 dosage unit in the apparatus, taking care to exclude air bubbles from the surface of the dosage unit and immediately operate the apparatus at the specified rate given in the individual monograph.
- Within the time interval specified, or at each of the times stated, withdraw a sample from a zone midway between the surface of the Dissolution Medium and the top of the rotating basket or blade, not less than 1 cm from the vessel wall.
- Replace the equivalence volume of the sample with equal volumes of fresh Dissolution Medium at 37°C.
- Perform the analysis as directed in the individual monograph using a suitable assay method.
- Repeat the test with additional dosage form units.
- If automated equipment is used for sampling or the apparatus is otherwise modified, verification that the modified apparatus will produce results equivalent to those obtained with the standard apparatus.
Dissolution Medium
A suitable dissolution medium is used. Use the solvent specified in the individual monograph. The volume specified refers to measurements made between 20° and 25°. If the Dissolution Medium is a buffered solution, adjust the solution so that its pH is within 0.05 units of the specified pH given in the individual monograph.
[NOTE— If dissolved gasses influence the dissolution results, dissolved gasses should be removed prior to testing]
Sampling Time
Where a single time specification is given, the test may be concluded in a shorter period if the requirement for minimum amount dissolved is met. samples are to be withdrawn only at the stated times within a tolerance of ± 2 %.
Acceptance Criteria
Dissolution test is pass if percentage of drug dissolved in each unit is not less than Q + 5 %. (Q is the labeled content of the dosage unit)
If test fail in first stage, Continue test with additional 6 unit and average of 12 units (S1 + S2) is equal to or greater than Q, and no unit is less than Q − 15%.
Continue testing through stage 3 with additional 12 unit and test pass if average of 24 units (S1 + S2 +S3) is equal to or greater than Q, not more than 2 units Q − 15%, and no unit is less than Q − 25%.
| Stage | Number Tested | Acceptance Criteria |
| S1 | 6 | Each unit is not less than Q + 5%. |
| S2 | 6 | Average of 12 units (S1 + S2) is equal to or greater than Q, and no unit is less than Q − 15%. |
| S3 | 12 | Average of 24 units (S1 + S2 +S3) is equal to or greater than Q, not more than 2 units Q − 15%, and no unit is less than Q − 25%. |
Dissolution test for Extended release dosage forms
Procedure Dissolution test
Proceed same as Immediate release dosage forms
Acceptance Criteria
Dissolution test is pass if percentage of drug dissolved in each unit is as per below table
| Level | Samples tested | Acceptance criteria |
| L1 | 6 | No individual value lies outside each of the stated ranges and no individual value is less than the stated amount at the final test time |
| L2 | 6 | The average value of the 12 dosage units (L1 + L2) lies within each of the stated ranges and is not less than the stated amount at the final test time; none is more than 10% of the label content outside each of the stated ranges; and none is more than 10% of label content below the stated amount at the final test time. |
| L3 | 12 | The average value of the 24 dosage units (L1 + L2 + L3) lies within the stated ranges and is not less than the stated amount at the final test time; not more than 2 of the 24 dosage units are more than 10% of label content outside each of the stated ranges; not more than 2 of the 24 dosage units are more than 10% of label content below the stated amount at the final test time; and none is more than 20% of label content outside each of the stated ranges or more than 20% of label content below the stated amount at the final test time |
Dissolution test for Delayed release dosage forms
Use Method A or Method B and the apparatus specified in the individual monograph.
Procedure for Method A
Acid Stage
- Place 750 mL of 0.1 N hydrochloric acid in the vessel and assemble the apparatus.
- Allow the medium to equilibrate to a temperature of 37 ± 0.5° C
- Place 1 dosage unit in the apparatus, cover the vessel, and operate the apparatus at the specified rate given in the monograph.
- After 2 hours of operation in withdraw sample and proceed immediately as directed under Buffer Stage.
- Perform an analysis of the sample using a suitable assay method.
Buffer Stage
- Add the buffer solution and adjusting the pH within 5 minutes.
- With the apparatus operating at the rate specified in the monograph, add to the fluid in the vessel 250 mL of 0.20 M tribasic sodium phosphate that has been equilibrated to 37 ± 0.5°. Adjust, if necessary, with 2 N hydrochloric acid or 2 N sodium hydroxide to a pH of 6.8 ± 0.05.
- Continue to operate the apparatus for 45 minutes, or for the specified time given in the individual monograph. At the end of the time period, withdraw a sample.
- Perform the analysis using a suitable assay method.
Procedure for Method B
Acid Stage
- Place 1000 mL of 0.1 N hydrochloric acid in the vessel, and assemble the apparatus.
- Allow the medium to equilibrate to a temperature of 37 ± 0.5°.
- Place 1 dosage unit in the apparatus, cover the vessel, and operate the apparatus at the rate specified in the monograph. After 2 hours of operation in 0.1 N hydrochloric acid, withdraw an aliquot of the fluid, and proceed immediately as directed under Buffer Stage
- Perform an analysis of the aliquot using a suitable assay method.
Buffer Stage
- For this stage of the procedure, use a buffer that previously has been equilibrated to a temperature of 37 ± 0.5°
- Drain the acid from the vessel,
- Add to the vessel 1000 mL of pH 6.8 phosphate buffer, prepared by mixing 0.1 N hydrochloric acid with 0.20 M tribasic sodium phosphate (3 : 1) and adjusting, if necessary, with 2 N hydrochloric acid or 2 N sodium hydroxide to a pH of 6.8 ± 0.05.
- [NOTE—This may also be accomplished by removing from the apparatus the vessel containing the acid and replacing it with another vessel containing the buffer and replacing it with another vessel containing the buffer and transferring the dosage unit to the vessel containing the buffer.]
- Continue to operate the apparatus for 45 minutes, or for the specified time given in the individual monograph. At the end of the time period, withdraw an aliquot of the fluid and perform the analysis using a suitable assay method
Acceptance Criteria
Acid stage: Dissolution test is pass if percentage of drug dissolved in each unit is as per below table
| Level | Samples tested | Acceptance criteria |
| A1 | 6 | No individual value exceeds 10% dissolved |
| A2 | 6 | Average value of the 12 dosage units (A1 + A2) is not more than 10% dissolved, and no individual value is greater than 25% dissolved. |
| A3 | 12 | Average value of 24 dosage units (A1 + A2 + A3) is not more than 10% dissolved, and no individual value is greater than 25% dissolved. |
Buffer stage: Dissolution test is pass if percentage of drug dissolved in each unit is as per below table
| Level | Samples tested | Acceptance criteria |
| B1 | 6 | No value is less than Q + 5% |
| B2 | 6 | Average value of the 12 dosage units (B1 + B2) is equal to or greater than Q, and no unit is less than Q – 15%. |
| B3 | 12 | Average value of the 24 dosage units (B1 + B2 + B3) is equal to or greater than Q; not more than 2 units are less than Q – 15%, and no unit is less than Q – 25%. |
Reference
- USP〈711〉 DISSOLUTION
what is Sink conditions?
Sink conditions are defined as the solution concentration corresponding to typically 5-10 times the nominal working concentration of the API in the dissolution medium.